2013年8月9日星期五
2013年8月8日星期四
2013年8月6日星期二
2013年8月5日星期一
specific surface area introduction--GOLD APP INSTRUMENTS
Specific surface area "SSA" is a property of solids which is the total surface area of a material per unit of mass, solid or bulk volume, or cross-sectional area.
It is a derived scientific value that can be used to
determine the type and properties of a material (e.g. soil). It is defined either by surface area divided by mass (with units of
m²/kg), or surface area divided by the volume (units of m²/m³ or m−1)
Measurement
The value obtained for specific surface area depends upon the method of
measurement. Several techniques have been developed to measure the specific
surface area of clays, including methylene blue (MB) stain test, ethylene
glycol monoethyl ether (EGME) method, Brenauer-Emmett-Teller (BET)adsorption method and Protein Retention (PR) method.
Calculation
The SSA can be simply calculated from a particle size distribution, making some assumption about the particle shape. This method, however,
fails to account for surface associated with the surface texture of the
particles.
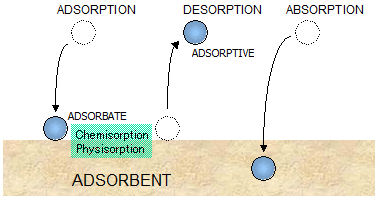
Adsorption
The SSA can be measured by adsorption using the BET isotherm. This has the advantage of measuring the surface of fine structures and
deep texture on the particles. However, the results can differ markedly
depending on the substance adsorbed.
Gas permeability
This depends upon a relationship between the specific surface area and the
resistance to gas-flow of a porous bed of powder. The method is simple and
quick, and yields a result that often correlates well with the chemical
reactivity of a powder. However, it fails to measure much of the deep surface texture.
Langmuir surface area introduction--GOLD APP INSTRUMENTS
1. The Langmuir Isotherm
Whenever a gas is in
contact with a solid there will be an equilibrium established between the
molecules in the gas phase and the corresponding adsorbed species (molecules or
atoms) which are bound to the surface of the solid.
As with all chemical
equilibria, the position of equilibrium will depend upon a number of factors :
- The relative stabilities of the
adsorbed and gas phase species involved
- The temperature of the system (both
the gas and surface, although these are normally the same)
- The pressure of the gas above the
surface
In general, factors
(2) and (3) exert opposite effects on the concentration of adsorbed species -
that is to say that the surface coverage may be increased by
raising the gas pressure but will be reduced if the surface temperature is
raised.
The Langmuir isotherm
was developed by Irving Langmuir in 1916 to describe the dependence of the
surface coverage of an adsorbed gas on the pressure of the gas above the
surface at a fixed temperature. There are many other types of isotherm (Temkin,
Freundlich ...) which differ in one or more of the assumptions made in deriving
the expression for the surface coverage; in particular, on how they treat the
surface coverage dependence of the enthalpy of adsorption. Whilst the Langmuir
isotherm is one of the simplest, it still provides a useful insight into the
pressure dependence of the extent of surface adsorption.
Important Note - Surface
Coverage & the Langmuir Isotherm
When considering
adsorption isotherms it is conventional to adopt a definition of surface
coverage (θ) which defines the maximum (saturation) surface coverage of
a particular adsorbate on a given surface always to be unity, i.e. θmax =
1 .
This way of defining
the surface coverage differs from that usually adopted in surface science where the more
common practice is to equate θ with the ratio of adsorbate species to surface
substrate atoms (which leads to saturation coverages which are almost
invariably less than unity).
2.
Langmuir Isotherm - derivation from
equilibrium considerations
We may derive the
Langmuir isotherm by treating the adsorption process as we would any other
equilibrium process - except in this case the equilibrium is between the gas
phase molecules (M), together with vacant surface sites, and the species
adsorbed on the surface. Thus, for a non-dissociative (molecular) adsorption
process we consider the adsorption to be represented by the following chemical
equation :
for further details, pls email to GOLD APP INSTRUMENTS
What Is The Difference Between Pore Size and Pore Size Distribution
Whereas
pore size is a measure of the diameter of the largest pore, pore size
distribution is a measure of the range of pore sizes. The range of pore sizes
can be normally distributed, and the spread can be quite narrow (e.g. the ratio
of largest to smallest may be less than 2). On the other hand, pore size
distribution can be very heterogeneous. In the case of large spreads and
heterogeneity, the pore size will be far less predictive of flow rate (either
filtration or capillary) than it will be for a membrane with a narrow pore size
distribution. It is important to note that the pore size corresponding to the
bubble point is not at the middle of the distribution, but is the largest pore.
2013年8月4日星期日
Zeilites physical characteristics
Zeolites are microporous,
aluminosilicate minerals commonly used as commercial adsorbents. The term
zeolite was originally coined in 1756 by Swedish mineralogist Axel Fredrik
Cronstedt, who observed that upon rapidly heating the material stilbite, it
produced large amounts of steam from water that had been adsorbed by the
material. Based on this, he called the material zeolite, from the Greek ζέω
(zéō), meaning "to boil" and λίθος (líthos), meaning
"stone".As of October 2012, 206 unique
zeolite frameworks have been identified, and over 40 naturally occurring
zeolite frameworks are known.Zeolites are widely used in
industry for water purification, as catalysts, for the preparation of advanced
materials and in nuclear reprocessing. They are used to extract nitrogen from
air to increase oxygen content for both industrial and medical purposes. Their
biggest use is in the production of laundry detergents. They are also used in
medicine and in agriculture.
订阅:
评论 (Atom)